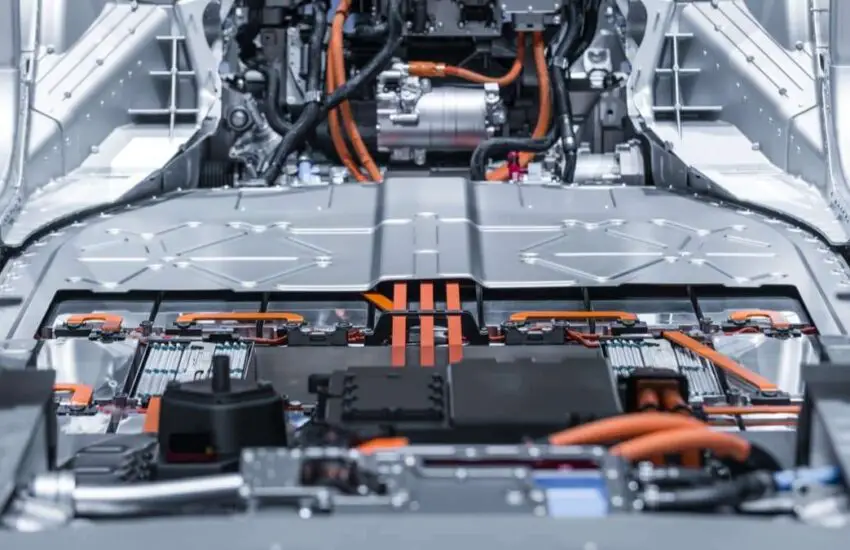
Why don’t gasoline and diesel cars use lithium-ion batteries? The Lithium-Ion battery, called Li-ion, is rechargeable. It contains lithium ions. These move from the anode to the cathode during discharge. During charging, they move from the cathode to the anode. There are different types of lithium-ion batteries with different levels of cathodes made from other lithium molecules and carbon anodes. The chemical reaction between the anode, cathode, and electrolyte generates an electric current.
Lithium-ion batteries in cars: how do they work?
Lithium batteries, also called Li-on batteries, or Lithium-on batteries, abbreviated as LIB, belong to the rechargeable battery type. An assembly composed of many cells, like lead-acid batteries and many other battery types. The battery uses lithium metal or a lithium alloy as the negative electrode material and uses a non-adhesive electrolyte solution.
Lithium batteries can be divided into two types: lithium metal batteries and lithium-ion batteries (Li-Ion batteries). Lithium-ion batteries do not contain metallic lithium and are rechargeable. The reason this type of battery is commonly used in electric vehicles is that the battery itself and the materials it’s made from contain a higher power density than other battery types, allowing people to make a small-sized battery but obtain a much larger capacity.
Most electric motorcycles and cars currently on the market are equipped with lithium-ion battery technology because this type of battery has a long lifespan and better performance but is more expensive than lead-acid batteries.
A Lithium battery is composed of four main components:
- Cathode: Determines the battery’s capacity and voltage and is the source of lithium ions.
- Anode: Allows current to flow in the external circuit and when the battery is charged, lithium ions are stored in the anode.
- Electrolyte: Acts as a conduit for lithium ions between the cathode and anode, formed from salts, solvents, and additives.
- Separator: A physical barrier that separates the cathode and anode.
Why don’t gasoline and diesel cars use lithium-ion batteries? – What you need to know here
The different classes of Li-ion batteries have not yet reached the automotive sector. These remain present in popular consumer electronics, including laptops and smartphones. The problem arises when using them in diesel or gasoline cars for transportation. We recommend searching online for the best maintenance tips to keep your car running without any problems.
Needs Protection
Lithium-ion batteries are not robust. These require additional protection against overcharging and rapid discharge. The current must be maintained within safety limits. The major advantage of Li-ion batteries is that they require protection circuits to keep them within safe operating limits. You wouldn’t prefer to have a battery that constantly leaks due to overcharging or malfunctions due to undercharging.

Battery Cost
The disadvantage of using Li-ion batteries for your car is their cost. These cost 40% more than lead-acid batteries. When looking to install a reliable power source for your vehicle, you don’t consider choosing a battery where cost is a significant issue. The Li-ion battery requires integrated circuits to manage and ensure that the voltage and current are within safety limits, which increases its cost. The lead-acid battery offers car owners a reliable and less expensive option for their car.
Sensitive to High Temperatures
The car’s Li-Ion battery is very sensitive to excessive heat. Overheating the device or overcharging the battery usually leads to more heat. Heat causes battery cell degradation faster than usual. When driving in an arid climate, having a lead-acid battery would make more sense.
Safety Concerns
The risks of Li-ion battery explosion due to overcharging or overheating are always high. The decomposition of electrolytes leads to gas formation. This can ignite the electrolyte and cause a fire. Transporting the batteries poses a significant risk to you, especially if you are shipping them in larger quantities. You wouldn’t risk installing a car battery with a high risk of explosion in the vehicle.

Summary
When deciding to replace the lead-acid battery with lithium-ion batteries that you are responsible for, make sure to read this blog to get a good idea.